Combinatorial chemistry
Combinatorial chemistry involves the rapid synthesis or the computer simulation of a large number of different but structurally related molecules or materials. It is especially common in CADD (Computer aided drug design) and can be done online with web based software, such as Molinspiration.
Contents |
Introduction
Synthesis of molecules in a combinatorial fashion can quickly lead to large numbers of molecules. For example, a molecule with three points of diversity (R1, R2, and R3) can generate 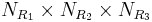 possible structures, where
possible structures, where 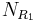 ,
, 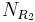 , and
, and 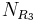 are the numbers of different substituents utilized.
are the numbers of different substituents utilized.
Although combinatorial chemistry has only really been taken up by industry since the 1990s, its roots can be seen as far back as the 1960s when a researcher at Rockefeller University, Bruce Merrifield, started investigating the solid-phase synthesis of peptides. Professor Pieczenik, a colleague of Nobel Laureate Merrifield, synthesized the first combinatorial library. US Patent 5,866,363. In the 1980s researcher H. Mario Geysen developed this technique further, creating arrays of different peptides on separate supports, but not a combinatorial library based on random synthesis.
In its modern form, combinatorial chemistry has probably had its biggest impact in the pharmaceutical industry. Researchers attempting to optimize the activity profile of a compound create a 'library' of many different but related compounds. Advances in robotics have led to an industrial approach to combinatorial synthesis, enabling companies to routinely produce over 100,000 new and unique compounds per year (see medicinal chemistry).
In order to handle the vast number of structural possibilities, researchers often create a 'virtual library', a computational enumeration of all possible structures of a given pharmacophore with all available reactants.[1] Such a library can consist of thousands to millions of 'virtual' compounds. The researcher will select a subset of the 'virtual library' for actual synthesis, based upon various calculations and criteria (see ADME, computational chemistry, and QSAR).
Materials Science
Materials science has applied the techniques of combinatorial chemistry to the discovery of new materials. This work was pioneered by P.G. Schultz et al. in the mid nineties[2] in the context of luminescent materials obtained by co-deposition of elements on a silicon substrate. His work was preceded by J. J. Hanak in 1970[3] but the computer and robotics tools were not available for the method to spread at the time. Work has been continued by several academic groups[4][5] as well as companies with large research and development programs (Symyx Technologies, GE, Dow Chemical etc.). The technique has been used extensively for catalysis,[6] coatings,[7] electronics,[8] and many other fields.[9] The application of appropriate informatics tools is critical to handle, administer, and store the vast volumes of data produced.[10] New types of Design of experiments methods have also been developed to efficiently address the large experimental spaces that can be tackled using combinatorial methods.[11]
Diversity-oriented libraries
Even though combinatorial chemistry has been an essential part of early drug discovery for more than two decades, so far only one de novo combinatorial chemistry-synthesized chemical has been approved for clinical use by FDA (sorafenib, a multikinase inhibitor indicated for advanced renal cancer).[12] The analysis of poor success rate of the approach has been suggested to connect with the rather limited chemical space covered by products of combinatorial chemistry. When comparing the properties of compounds in combinatorial chemistry libraries to those of approved drugs and natural products, Feher and Schmidt[13] noted that combinatorial chemistry libraries suffer particularly from the lack of chirality, as well as structure rigidity, both of which are widely regarded as drug-like properties. Even though natural product drug discovery has not probably been the most fashionable trend in pharmaceutical industry in recent times, a large proportion of new chemical entities still are nature-derived compounds, and thus, it has been suggested that effectiveness of combinatorial chemistry could be improved by enhancing the chemical diversity of screening libraries. As chirality and rigidity are the two most important features distinguishing approved drugs and natural products from compounds in combinatorial chemistry libraries, these are the two issues emphasized in so-called diversity oriented libraries, i.e. compound collections that aim at coverage of the chemical space, instead of just huge numbers of compounds.
Patent classification subclass
In the 8th edition of the International Patent Classification (IPC), which entered into force on January 1, 2006, a special subclass has been created for patent applications and patents related to inventions in the domain of combinatorial chemistry: "C40B".
See also
- Dynamic combinatorial chemistry
- Cheminformatics
- Drug discovery
- High-throughput screening
- Mathematical chemistry
- Combinatorial biology
- Molecular modeling
References
- ^ E. V.Gordeeva et al. "COMPASS program - an original semi-empirical approach to computer-assisted synthesis" Tetrahedron, 48 (1992) 3789.
- ^ X. -D. Xiang et al. "A Combinatorial Approach to Materials Discovery" Science 268 (1995) 1738
- ^ J.J. Hanak, J. Mater. Sci, 1970, 5, 964-971
- ^ H. Koinuma et al. "Combinatorial solid state materials science and technology" Sci. Technol. Adv. Mater. 1 (2000) 1 free download
- ^ Andrei Ionut Mardare et al. "Combinatorial solid state materials science and technology" Sci. Technol. Adv. Mater. 9 (2008) 035009 free download
- ^ Applied Catalysis A, Volume 254, Issue 1, Pages 1-170 (10 November 2003)
- ^ , J. N. Cawse et. al, Progress in Organic Coatings, Volume 47, Issue 2, August 2003, Pages 128-135
- ^ Combinatorial Methods for High-Throughput Materials Science, MRS Proceedings Volume 1024E, Fall 2007
- ^ Combinatorial and Artificial Intelligence Methods in Materials Science II, MRS Proceedings Volume 804, Fall 2004
- ^ QSAR and Combinatorial Science, 24, Number 1 (February 2005)
- ^ J. N. Cawse, Ed., Experimental Design for Combinatorial and High Throughput Materials Development, John Wiley and Sons, 2002.
- ^ D. Newman and G. Cragg "Natural Products as Sources of New Drugs over the Last 25 Years" J Nat Prod 70 (2007) 461
- ^ M. Feher and J. M. Schmidt "Property Distributions: Differences between Drugs, Natural Products, and Molecules from Combinatorial Chemistry" J. Chem. Inf. Comput. Sci., 43 (2003) 218
External links
- IUPAC's "Glossary of Terms Used in Combinatorial Chemistry"
- ACS Combinatorial Science (formerly Journal of Combinatorial Chemistry)
- Combinatorial Chemistry Review
- Molecular Diversity
- Combinatorial Chemistry and High Throughput Screening
- Combinatorial Chemistry: an Online Journal
- SmiLib - A free open-source software for combinatorial library enumeration
- GLARE - A free open-source software for combinatorial library design